Model No: 124433-A12
- Best Quality
- Affordable Pricing
- On-Time Delivery
- Customer Satisfaction
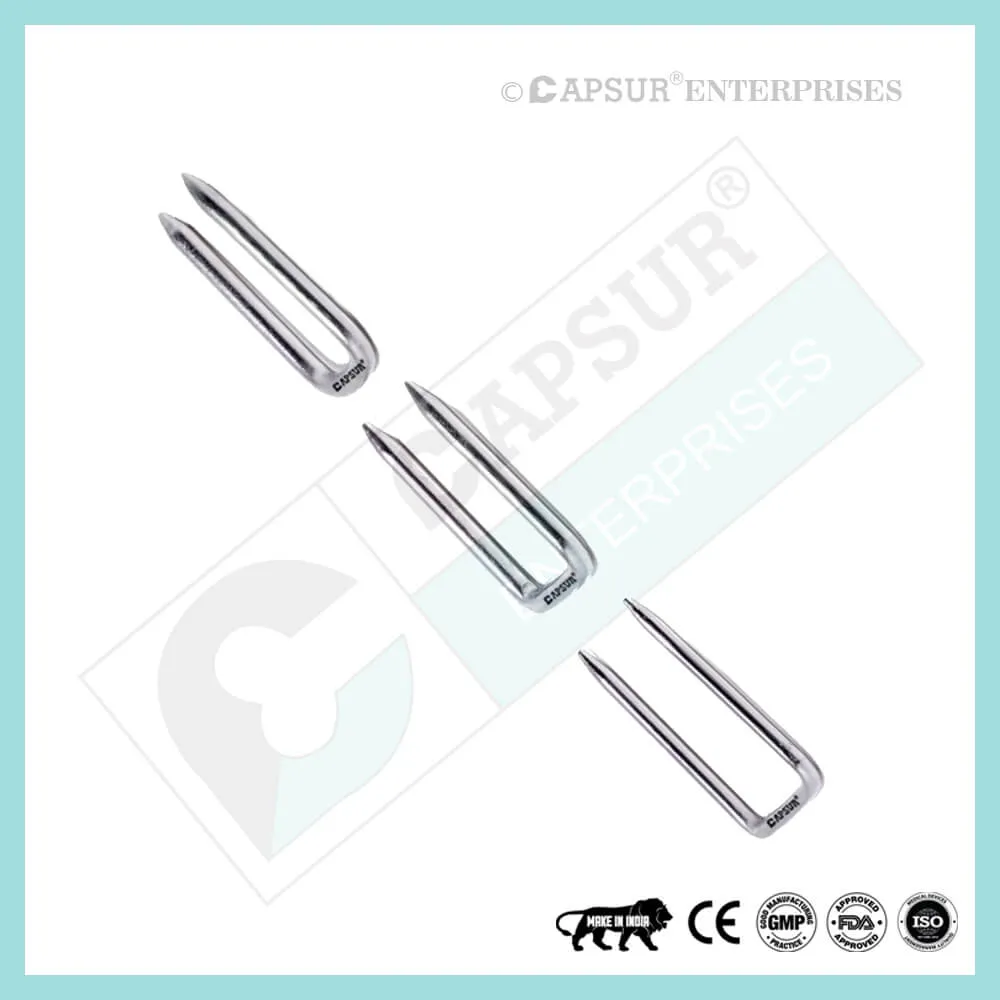
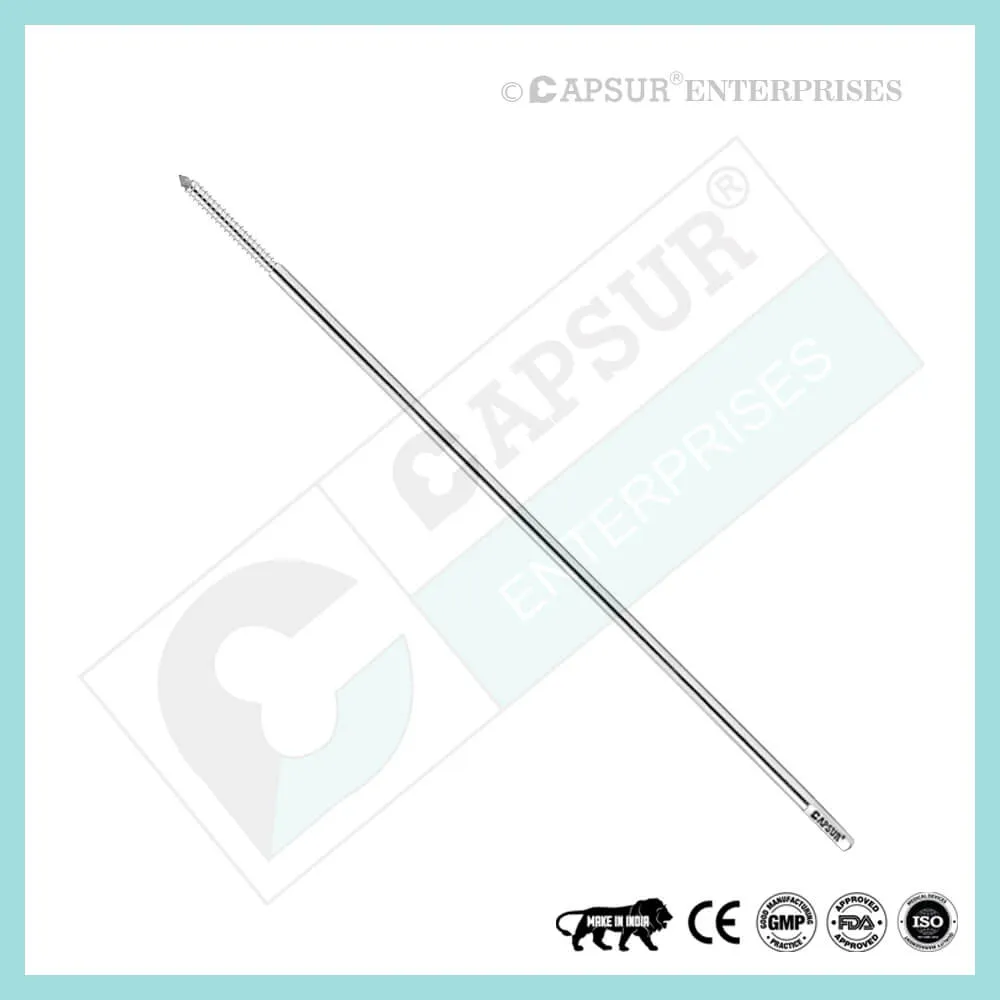
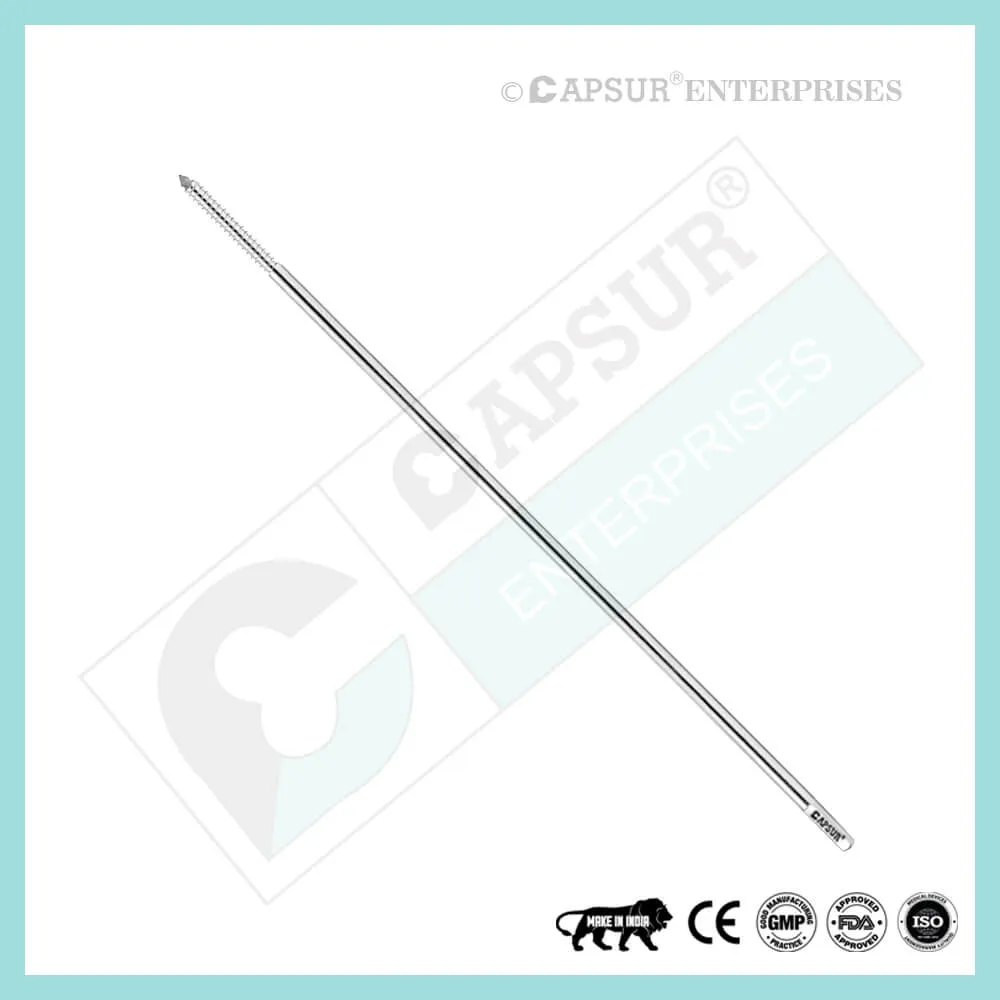
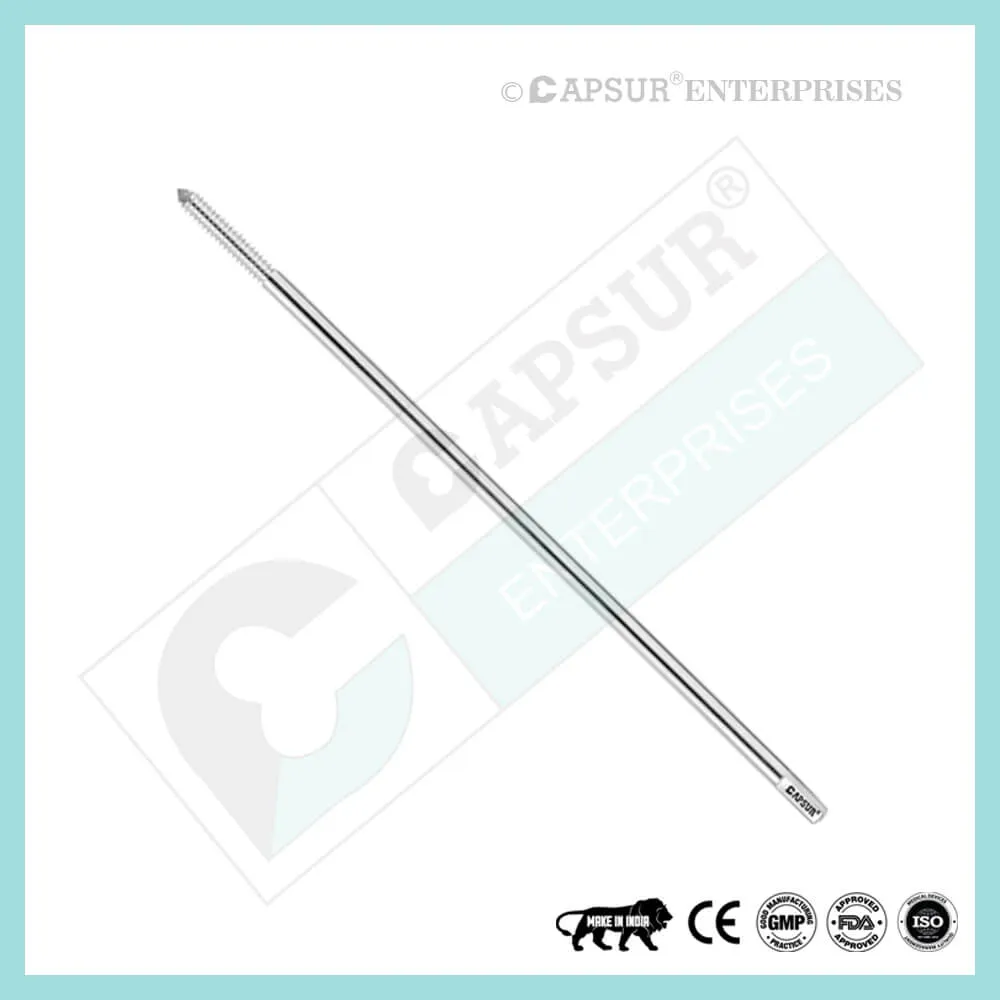
Specification of Epiphyseal Staple
Knee angular deformity is treated with epiphyseal staples. Each side of the epiphysis should have two or more Epiphyseal Staples.
To ensure the highest quality, our epiphyseal staples are made from the best medical-grade materials available. Epiphyseal staples come in various sizes, including:
- Dia(A) x Length(B) x Length(C) = 1.5 mm x 16 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 1.5 mm x 22 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 1.5 mm x 26 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.0 mm x 16 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.0 mm x 22 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.0 mm x 26 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.5 mm x 16 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.5 mm x 22 mm x 19 mm
- Dia(A) x Length(B) x Length(C) = 2.5 mm x 26 mm x 19 mm
Other Important Info of Epiphyseal Staple
Epiphyseal Staple for angular deformity at the knee
Epiphyseal staples were used to treat 56 patients with angular deformities of 82 knees between 1954 and 1973, and the patients were then monitored until they reached adulthood. Sixty-four knock-knees and eighteen bowlegs were present. Long legs were stapled asymmetrically in twelve patients with concurrent leg-length discrepancies. Before the Epiphyseal Staples were removed, the deformities were given time to overcorrect, but the rebound phenomenon manifested in twenty-two patients with thirty-five deformities.
When the legs appeared straight in older kids, the epiphyseal staple was removed. Exaggerated physiological deformities might naturally heal. Before girls and boys reach the skeletal age of eleven and twelve, respectively, they shouldn’t be stapled. Early correction is given to secondary deformities. Significant difficulties were nonexistent. Epiphyseal Staples had to undergo ten revisions as a result of extrusion or shifting. In 87% of the deformities, the results were satisfactory or improved. When necessary, epiphyseal stapling is a secure and reliable way to treat children’s growing knee angular deformity.
Control of bone growth by epiphyseal staple
The elongation of bone at the distal femoral and proximal tibial epiphyses is abruptly and almost completely stopped by effective stapling.
Compared to other operative methods of controlling bone growth, this procedure is less involved and carries a lower risk of complications.
Angular deformity may be fixed while the child is growing. It takes little time to correct knock-knee, bowleg, back-knee, flexion deformities, or combinations of these deformities.
If growth after stapling occasionally exhibits complicating irregularities, these should be clinically identified and corrected right away by rearranging the staples.
Growth at the epiphysis resumes at roughly the same rate as would be anticipated on the opposite side, if both sides were normal, after the epiphyseal staples are removed. It fluctuates between being faster and slower. Other factors than epiphyseal staples are typically to blame for the variations in rate.
The persistence of a straight extremity following the correction of an idiopathic knock-knee is the best indication of the normal rate of growth following removal of epiphyseal staples.
Epiphyseal Staple Risk Factor
When assessing the prognosis in each case, contraindications—which may be partial or complete—must be taken into account. Under the following circumstances, alternative management strategies may need to be taken into account:
- infections that are systemic or local, acute or chronic.
- either localized, systemic, or chronic inflammation.
- serve as a dangerous vascular, nervous, or muscular disease.
- Bone defects that would prevent the implant from being properly anchored.
- All associated illnesses that might jeopardize the implant’s success and functionality.
Warnings and Precautionary for Epiphyseal Staple
The surgeon and support staff should read the safety instructions in this document as well as any product-specific information in the product description, surgical techniques, and/or brochures before using Epiphyseal Staple.
Epiphyseal staples are designed, built, and produced with the utmost care using materials of the highest quality. If used properly, these high-quality epiphyseal staples guarantee the best possible working outcomes. As a result, the usage guidelines and safety advice below must be followed.
Epiphyseal staples should only be used as directed in order to avoid injury to the operator, patients, or other people as well as tissue damage, premature wear, and instrument destruction.
The operating surgeon must actively participate in the medical care of their patients. The surgeon must have a complete understanding of the instruments, their limitations, and the surgical procedure. The surgeon and the surgical team are responsible for exercising caution in the selection and use of surgical instruments. Epiphyseal staples should only be used after adequate surgical training has been completed.
Factors that could harm the operation’s success include:
- allergies to materials implanted.
- regional bone tumors.
- osteomalacia or osteoporosis.
- metabolic disturbances and systemic disease.
- drug and alcohol abuse.
- Excessive shock-producing physical activity that exposes the implant to blows and/or heavy loads.
- Patients who lack the mental capacity to comprehend and follow instructions from a doctor.
- Unhealthy overall.
- Potential Negative Effects
The most frequent side effects following implantation are as follows:
- Implant loosening that may be caused by the implant reacting with the tissue or by the fixation site being loaded repeatedly.
- the two stages of infection.
- additional bone fracture brought on by abnormal stress or weakened bone structure.
- a hematoma or pressure-related pressure that causes temporary or permanent neural damage.
- Hematomas from wounds and slow wound healing.
- Venous thrombosis, pulmonary embolism, and cardiac arrest are examples of vascular disease.
- heterotopically ossifying.
- discomfort and pain brought on by the implant.
- Implant mechanical failure, such as bending, loosening, or breakage.
- Implant migration leading to injury.
Preoperative Planning for Epiphyseal Staple
Following a thorough clinical evaluation of the patient, the operation is planned. X-rays are also necessary to provide a clear picture of the bony anatomy and any associated deformities. The entire set of implants must be available at the time of the operation, along with the appropriate implantation tools.
The patient should be informed of any risks or side effects that could arise from using an epiphyseal staple by the clinician. If the patient has allergies to any of the implant materials, it is crucial to know this before surgery. Additionally, the patient needs to be made aware that the device’s performance cannot be guaranteed because problems may reduce its lifespan.
Epiphyseal Staple Precautions
During reprocessing, verify that the instruments are functional and look for wear. Before using, replace any worn-out or broken instruments.
It is advised to use the tools designated for this screw.
Use caution when handling equipment, and put used bone-cutting tools in a sharps container.
Always use suction and irrigation to remove any debris that may be produced during implantation or removal.
Epiphyseal Staple Warnings
When put through excessive force while being used, epiphyseal staples can break. We advise that the broken part be removed whenever possible and practical for the particular patient, though the surgeon will ultimately decide whether to do so based on the risk involved. Be aware that implants lack the natural bone’s strength. Significant loads may cause implants to fail.
The user’s glove or skin may be pinched or torn by the sharp edges or moving joints of some instruments, screws, and cut plates.
Be sure to get rid of any fragments that weren’t fixed during surgery.
While the surgeon will ultimately decide whether to remove the implant, we advise that fixation devices be taken out as soon as it is safe and practical for the specific patient and after their purpose as a healing aid has been fulfilled. To prevent refracture, implant removal should be followed by adequate post-operative care.
Epiphyseal Staple General Adverse Events
There are risks, side effects, and adverse events associated with all major surgical procedures. While there are many possible reactions, the following are some of the most frequent ones: issues related to anesthesia and patient positioning (such as nausea, vomiting, dental injuries, neurological impairments, etc.), thrombosis, embolism, infection, damage to nerve and/or tooth roots or other critical structures, such as blood vessels, excessive bleeding, damage to soft tissues, including swelling, abnormal scar formation, functional impairment of the musculoskeletal system, and pain.
| Epiphyseal Staple |
|---|